第五届“维生素D争议”国际会议
——老年人群中的维生素D:共识声明
Vitamin D in the older population: a consensus statement
Giustina A, Bouillon R, Dawson-Hughes B, etal.
Endocrine. 2023 Jan;79(1):31-44.
介绍
第五届“维生素D争议”国际会议于2021年9月15日至18日在意大利Stresa举行,这是2017年开始的系列会议之一。国际专家和领导人参加了本次会议,旨在回顾和讨论有关维生素D的争议性话题。四场会议讨论了维生素D的不同主要方面:衰老、胃肠道系统、指南和COVID-19。会前参与者回顾了他们指定主题的现有文献,并在会议期间进行了充分的讨论以达成共识。本文总结了专家们对衰老与维生素D的讨论。这部分回顾的文章类型没有限制,优先考虑随机临床试验;没有这些资料时,会考虑观察、实验或意见研究。此外,研究中“老年人口”的年龄界限从>65岁到>75岁各不相同,其中以>65岁为界限的居多。
年龄对维生素D的影响
维生素D的产生和代谢都会随着衰老而变化,原因包括:皮肤产生维生素D的能力降低和阳光照射减少、刺激胃肠道钙吸收的维生素D相对抵抗以及肾功能下降(衰老的肾脏通过25-羟基维生素D(25[OH]D)产生1,25-二羟基维生素D的能力也较差)。此外,吸烟、较高的身体脂肪百分比也可能会导致血清25(OH)D浓度降低。
1)皮肤产生维生素D的能力降低
皮肤样本显示,从20岁到80岁,7-脱氢胆固醇的浓度下降了50%以上,老化皮肤产生的维生素D大约比年轻皮肤少40%。Chalcraft及其同事通过十年为自变量和对数D3产量的简单线性回归模型算出:与年龄相关的维生素D3产量每十年减少13%,表明70岁时的维生素D3产量仅为20岁时的一半(图1)。当然,皮肤中维生素D生成的潜力取决于一系列因素,季节、一天中的时间、纬度、海拔、云量、空气污染、皮肤类型、衣服、防晒霜和生活方式等都会影响太阳UV-B能量刺激皮肤合成维生素D的能力。
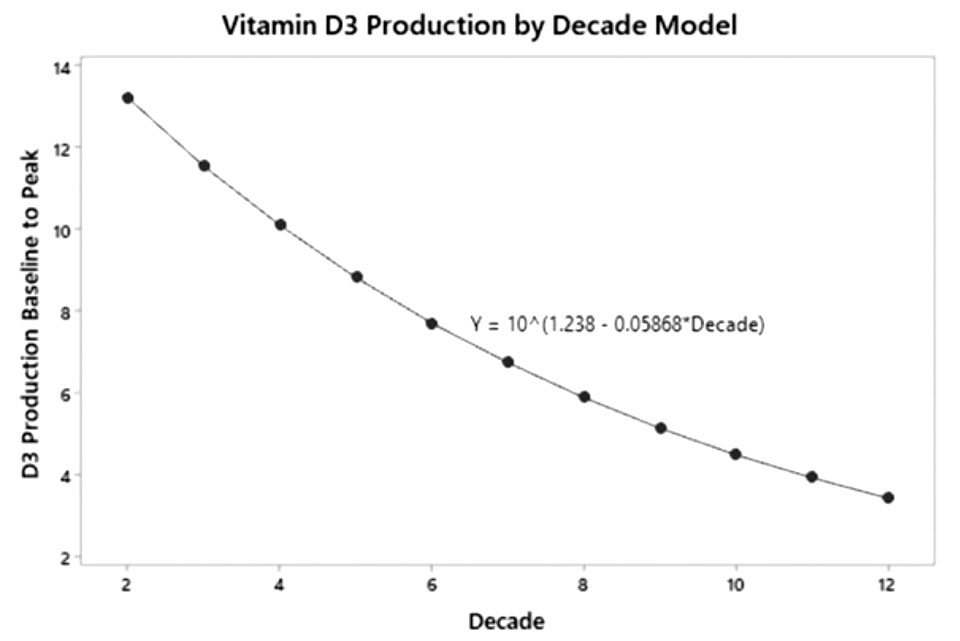
图1. 维生素D3生产年龄连续模型
2)阳光照射减少
在皮肤中,波长为290-315nm的太阳紫外线(UV-B)会将7-脱氢胆固醇转化为前维生素D3。单次15分钟的阳光照射(>40%身体面积)会导致皮肤产生大量维生素D。虽然衰老可能会减少皮肤合成,阳光照射仍然是维生素D3的重要来源。随着年龄的增长,维生素D的产生量会减少,但皮肤的生产储备能力是充足的。因此,大多数老年人可能可以从阳光中产生足够的维生素D,但居家人士仍然需要补充维生素D才能满足维生素D需求。
经证实,每周三次,在背部1000cm2的表面上以最小红斑剂量的一半进行人工紫外线照射,可在3个月内使平均血清25(OH)D从25nmol/L增加到60nmol/L,与每日口服维生素D3 400IU的效果相似。这一观察结果表明,在这些条件下,每天1000cm2的皮肤暴露可能导致血清25(OH)D的增加,相当于每天补充约800IU的维生素D(>70岁人群的每日推荐量)。在另一项研究中,8名缺乏维生素D的老年精神科患者每周接受一次半身紫外线照射,照射剂量为最小红斑剂量的一半(2分钟),持续8周。中位血清25(OH)D从基线时的26.5nmol/L(范围12-58)增加至43.5nmol/L(范围36-71)。皮肤表面积超过15000cm2,理论上皮肤中可能产生的维生素D量可能很高,即使是老年患者,紫外线照射也能有效触发皮肤维生素D的合成。
维生素D缺乏对老年人的影响
1.骨骼
严重的维生素D缺乏会对骨骼产生不利影响,包括骨软化、高骨转换和骨质流失,以及老年人髋部骨折的风险增加(图2)。维生素D是一种阈值营养素,在严重缺乏维生素D的个体中,维生素D可以降低老年人骨折风险;轻度维生素D缺乏会导致骨质疏松症的发生,但单独补充维生素D似乎并不能减少骨折。Chapuy等人研究发现,法国老年非卧床疗养院和公寓妇女在18个月内,每日补充维生素D(800IU)和钙(1200mg),髋部和其他非椎骨骨折分别减少43%和32%。在该人群中,平均基线血清25(OH)D浓度仅为36nmol/L。然而,测试采用的是老式竞争性蛋白质结合法,测值比高效液相色谱法高80%。高效液相色谱交叉校准浓度平均值约为20nmol/L,表明大多数参与者患有中度或重度维生素D缺乏症。
严重缺乏维生素D的个体甲状旁腺激素(PTH)水平会升高,这种继发性甲状旁腺功能亢进症可能导致骨质流失和骨折风险的病理生理学变化。两项较早的研究提供了可参考的25(OH)D阈值,低于该阈值PTH水平可能会上升。在MORE试验中,与血清25(OH)D>50nmol/L(3.5±1.5pmol/L)的女性相比,两组不同程度维生素D缺乏的女性血清PTH较高(血清25(OH)D<25nmol/L和25(OH)D 25-50nmol/L;PTH分别为4.8±2.2和4.1±1.8pmol/L)。血清25(OH)D>50nmol/L组血清PTH无明显下降,维生素D处理后两组血清PTH均显著降低。一项基于LASA内收集数据的研究发现25(OH)D的阈值范围为40-60nmol/L。在另一项针对绝经后骨质疏松妇女的研究中,PTH继发性升高的阈值也出现在血清25(OH)D 50nmol/L浓度时。关于25(OH)D的精确水平仍然存在一些争议,如果能够清楚地确定这样一个阈值,人们就可以通过维生素D缺乏引起的继发性甲状旁腺功能亢进的程度来定义维生素D缺乏的程度。然而,这个阈值也可能取决于其他因素,如钙摄入量和体力活动。
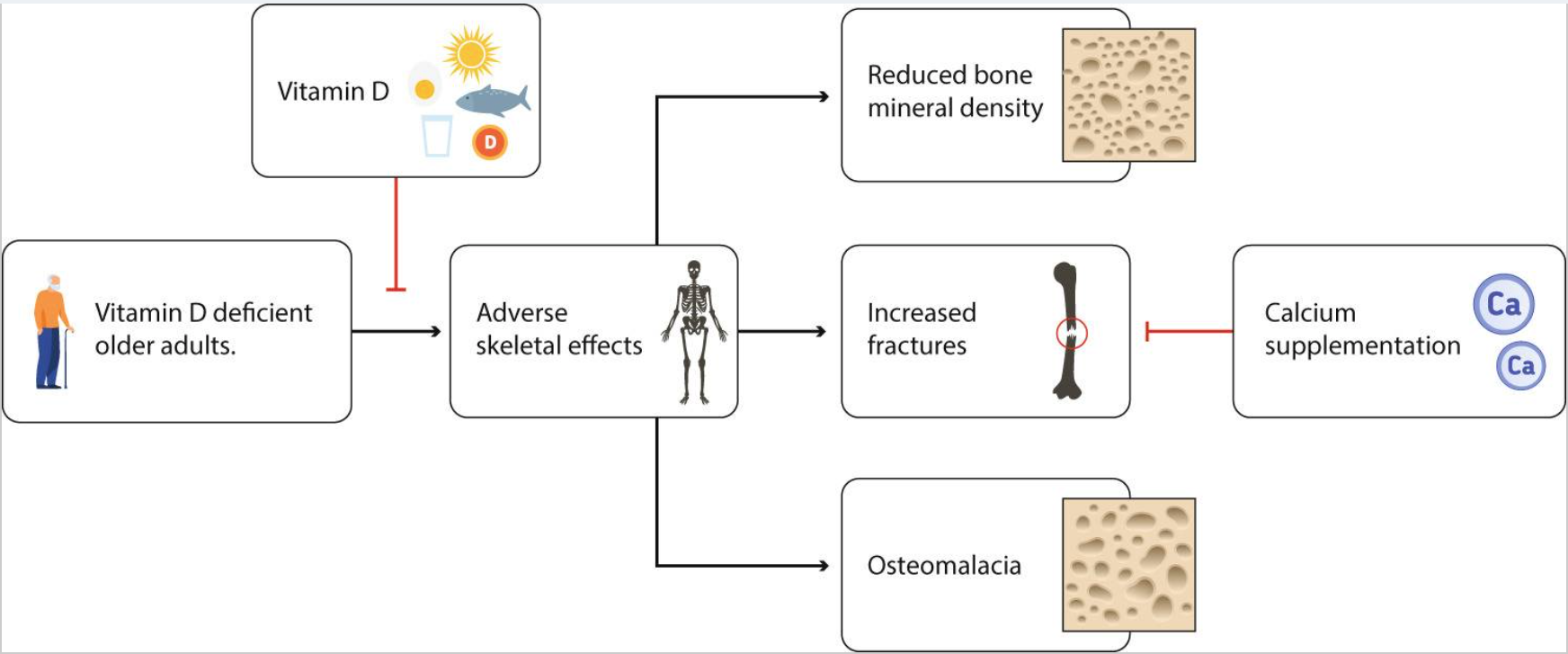
图2. 老年人维生素D缺乏对骨骼的影响以及维生素D在预防中的作用
2.骨密度
新西兰维生素D评估(ViDA)研究的一项针对老年社区居住男性和女性的研究表明,连续2年每月服用100000IU维生素D,平均基线血清25(OH)D水平为55nmol/L的人群股骨颈和髋部骨质流失无改善。基线血清25(OH)D<30nmol/L的人群,脊柱、股骨颈和全髋骨丢失有所改善,并且在脊柱和股骨颈处具有统计学显著性。相比之下,基线血清25(OH)D>30nmol/L的患者仅全髋部骨质流失,减少幅度较小。在ViDA研究的整个队列中,平均基线血清25(OH)D浓度为66nmol/L且钙摄入量充足,补充维生素D3超过3.3年并没有降低骨折的发生率。
在第二项研究,即Aberdeen研究中,在冬末招募了305名绝经后妇女,并随机让她们接受维生素D 400IU/天、1000IU/天或安慰剂,为期一年。事后分析显示,维生素D 1000IU/天对基线25(OH)D≤30nmol/L的患者的脊柱和髋部BMD具有显著改善作用,但对基线25(OH)D高于这一水平的患者没有显著影响。血清25(OH)D水平<30nmol/L的老年人最有可能出现骨骼负面影响,而补充维生素D有明显的改善作用。
3.跌倒
Meta分析的结果相互矛盾,但适度剂量的维生素D(每天700-1000IU)似乎可以降低缺乏维生素D的老年人跌倒的风险。相反,不频繁的较大剂量补充可能会增加跌倒风险。在基线25(OH)D水平<50nmol/L的绝经后妇女中进行的一项随机前瞻性、安慰剂对照的多剂量维生素D试验发现,低剂量维生素D治疗可降低跌倒的风险,而每天4000和4800IU的剂量可增加跌倒的风险。因此,每日应低至中剂量的维生素D补充,避免每日或间歇性高剂量补充。
4.骨骼外效应
维生素D被代谢成大约50种代谢物,它的活性形式1,25(OH)2D为维生素D受体(VDR)的配体。VDR在大多数组织中表达,它的普遍存在是维生素D具有许多骨骼外作用这一假设的基础,这一主题在小组之前会议上最近发表的几篇文章中进行了回顾(图3)。
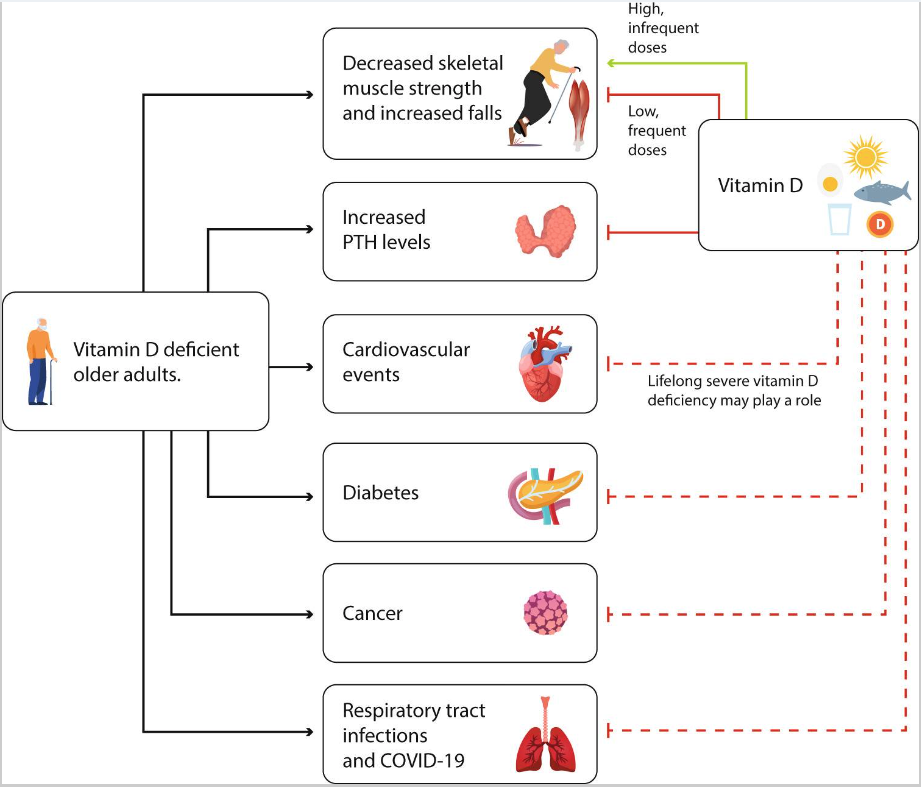
图3. 老年人缺乏维生素D对骨骼的额外影响以及维生素D的预防作用
注:实线表示已知的效果,虚线表示可能的影响。
5.骨骼肌
一般认为维生素D缺乏影响两种不同的肌肉骨骼途径,一种涉及神经肌肉组织的影响,导致跌倒和骨折;另一种通过钙吸收减少,导致甲状旁腺激素(PTH)水平升高,骨吸收增加,骨质流失导致骨折风险增加。维生素D和钙缺乏时,可能导致循环甲状旁腺激素水平的增加,对肌肉造成直接的不良影响。临床前和临床证据表明甲状旁腺激素对肌肉有直接影响,完整的牛PTH和合成的PTH1-34片段增加了大鼠骨骼肌的肌肉降解和新合成的丙氨酸和谷氨酰胺的释放。
临床上,晚期原发性甲状旁腺功能亢进患者有神经肌肉体征和症状以及肌肉无力,甲状旁腺手术成功后肌肉无力很快逆转。两组老年妇女具有相似的临床特征,25(OH)D水平低,但PTH水平不同(高于或在范围内),在几项肌肉力量和功能测试中表现不同。PTH水平较高的组膝关节屈曲强度较低,最大肌力产生较低,姿势稳定性降低。一项小型观察性研究,调查了83名平均年龄为84岁的养老院居民{25(OH)D平均水平为27nmol/L、PTH中位水平为5.2pmol/L(参考范围1-6.5pmol/L)},评估了甲状旁腺激素在跌倒中的作用。在此期间,33名参与者至少跌倒一次。那些跌倒的人有较低的25(OH)D水平和较高的甲状旁腺激素水平,而两组的1,25(OH)2D水平没有显著差异。Logistic回归分析表明甲状旁腺激素水平是跌倒的独立决定因素。Sambrook等人对637名普遍缺乏维生素D的老年人进行了一项大型观察性研究,这些老年人平均年龄为86岁,居住在澳大利亚的中等全面护理机构。跌倒者和非跌倒者血清25(OH)D水平分别为28.8和33.2nmol/L,PTH水平分别为64.8和57.0pg/ml。逻辑回归显示甲状旁腺激素是跌倒的独立预测因子。每天低剂量的维生素D可能会降低缺乏维生素D的老年人跌倒的风险,进一步检查跌倒的独立预测因子——甲状旁腺激素也是必要的。
6.心血管疾病
临床前数据表明缺乏维生素D与心血管风险有关。实验表明,缺乏VDR可引起高肾素高血压、心肌肥大和纤维化、血栓形成增强。两项大型随机对照试验(VIDA和VITAL)清楚地表明,补充维生素D不会减少心血管疾病,VIDA试验显示补充维生素D对中心血压有轻微的改善作用。
孟德尔随机化(MR)研究没有发现遗传性低血清25(OH)D浓度与CV疾病之间的联系,但这些研究中的综合多态性不允许任何大于5%的血清25(OH)D变异的预测值。最近一项大型磁共振研究的总体结果证实了这一结论,但通过结合磁共振和血清25(OH)D水平来看,严重缺乏维生素D组血清25(OH)D的遗传水平较低(<10 ng/mL)会增加CV疾病和死亡率。维生素D状态不是心血管疾病负担的主要因素,但终身严重缺乏维生素D可能起心血管疾病。
7.癌症
许多受维生素D内分泌系统调控的基因参与调控细胞周期和细胞分化。动物和临床前数据表明,维生素D作用的完全缺乏易导致癌症,且在早期补充维生素D可预防癌变。维生素D缺乏与许多癌症有关,尤其是结肠癌、乳腺癌和前列腺癌。两个主要的大型随机对照试验(VITAL和VIDA)没有发现长期补充维生素D对癌症发病率的影响。然而,根据VITAL、四项类似的Meta分析研究及D-health研究,发现每天补充2000IU维生素D的患者,癌症死亡率显着降低;每月高剂量(60000IU)维生素D补充的患者癌症的死亡风险增加。
8.糖尿病
许多临床前和观察性研究表明,维生素D水平低与2型糖尿病(T2D)之间存在联系。两项专门用于预防糖尿病的试验表明,与安慰剂相比,补充维生素D可使未选择维生素D缺乏症的糖尿病前期患者患糖尿病的风险降低10-13%。这与最近的两项Meta分析结论一致,即补充维生素D可使T2D进展的风险降低约10%,特别是在使用剂量超过1000IU/天且非肥胖受试者中。然而,在维生素D与2型糖尿病研究(D2d)的大型随机对照试验中,补充维生素D仅显示出减缓糖尿病前期向T2D进展的非显著趋势(0.88CI为0.75-1.04;P=0.12)。
维生素D和钙
维生素D的膳食来源很少。大多数采用西方饮食的体弱老年人每天的维生素D摄入量较低(即约150IU),他们的阳光照射和皮肤维生素D合成能力受到限制,含钙食物也可能摄入不足。随机对照试验(RCT)的Meta分析表明,维生素D与钙结合使用且依从率>80%时,可使髋部骨折和其他非椎骨骨折的发生率分别降低16%和14%。与60-70岁的人相比,70-80岁以上的人对骨折的影响更大。Yao及其同事最近进行的一项Meta分析得出了类似的结论,维生素D可使任何骨折的风险降低6%,髋部骨折的风险降低16%,但前提是同时服用钙补充剂。因此,大多数老年人应该补充维生素D和钙。
预防维生素D缺乏的策略
当前各国面临的一个共问题是如何实施公共卫生政策来预防老年人维生素D缺乏。除了充足的饮食之外,关键是阳光照射、食物强化及补充剂(图4)。
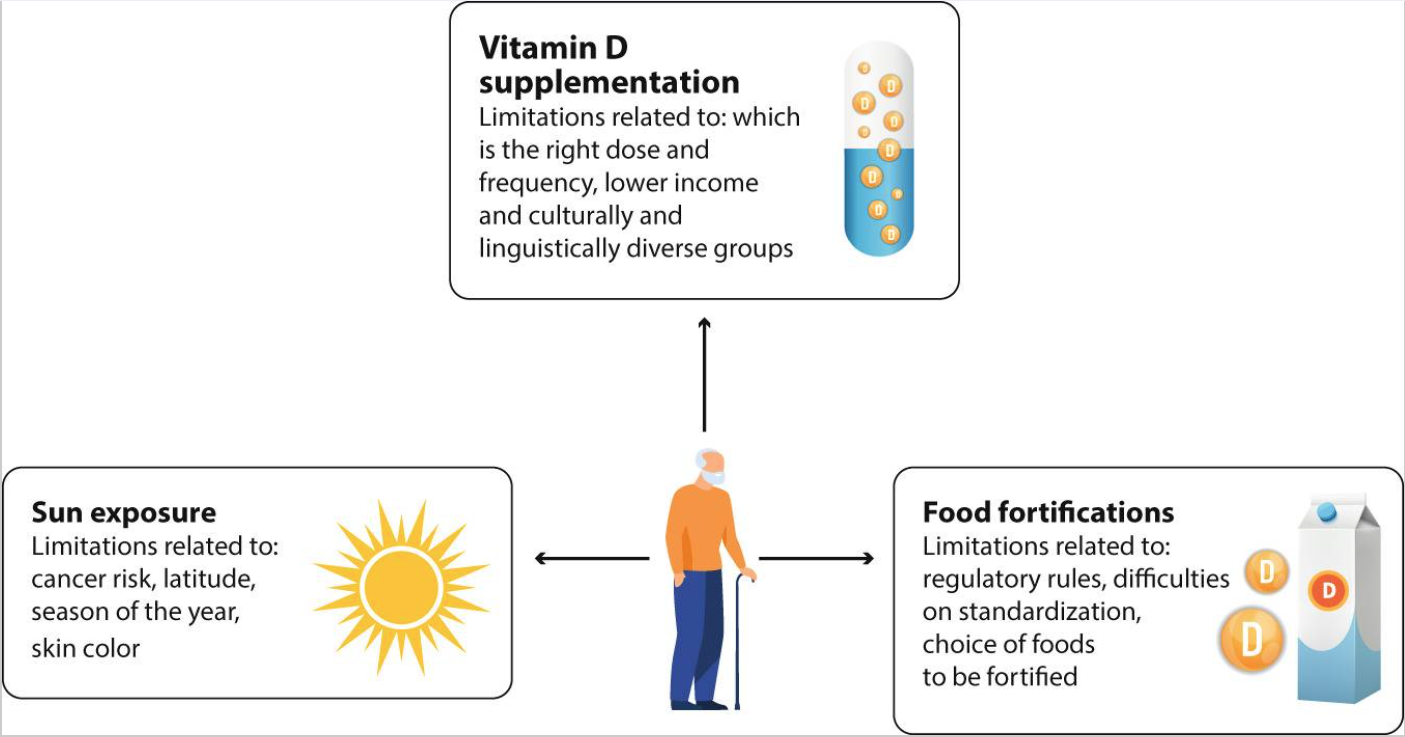
图4. 老年人避免维生素D缺乏的策略及相关限制
1.阳光照射
增加紫外线照射来提高维生素D水平是有争议的。世界卫生组织认为,紫外线照射造成的健康威胁可能超过维生素D缺乏造成的健康风险。非黑色素瘤皮肤癌占全球所有癌症的三分之一,主要存在于老年人群中,紫外线照射是其主要原因。尽管这一策略存在争议,但应该清楚地认识到,血清25(OH)D水平在世界范围内存在季节性变化,夏季后增加。阳光充足的国家维生素D含量较高,佝偻病和骨软化症的患病率较低。年龄和阳光照射是影响血清25(OH)D水平的重要因素。户外活动被视为预防严重维生素D缺乏、改善健康的合理措施,但人们必须了解过度日晒的风险,以避免晒伤、防晒霜的使用以及如何及早识别癌性皮肤病变。
2.食品和食品强化
在过去3年中,发表了两项重要声明,一项来自关于维生素D的二等奖基金论坛,一项来自欧洲钙化组织协会。这些强调了公共卫生目标是降低佝偻病和骨软化症的风险,并避免血清25(OH)D水平低于25nmol/L。食物中维生素D的天然来源很少(西方饮食中每天约为150IU)。因此,在食品中添加维生素D是一种有效的策略,适用于大量人群以避免严重缺乏,特别是对于阳光照射有限的国家。加拿大,牛奶中维生素D强化是强制性的,与英国、美国和德国相比,血清水平低于25nmol/l的发生率较低。
3.补充维生素D
补充维生素D似乎是有效实现维生素D充足的最简单方法。大多数已测试的方案均显示血清25(OH)D水平呈剂量依赖性增加,但存在显著个体差异。结合强化和补充来解决维生素D缺乏问题,理想情况下可将整个人群的血清25(OH)D水平提高至50nmol/L。一些使用间歇性高剂量维生素D补充剂的研究表明,跌倒和骨折的风险会增加,因此优选每日或每周补充剂量。
Cashman及其同事根据7项基于冬季的随机对照试验(包括882项)的Meta回归分析,计算了达到血液中目标血清25(OH)D水平的每日剂量,参与者年龄从4岁到90岁。结论是避免严重缺乏(即97.5%的个体达到25nmol/L)的每日剂量是400IU。每天则需要1000IU才能达到50nmol/L的安全水平;然而,每天1000IU的剂量比医学研究所(IOM)和其他监管机构先前推荐的剂量要高。
总结
老年人群中应避免血清25(OH)D<30nmol/L,以防维生素D缺乏会对骨骼产生影响,如BMD下降、继发性甲状旁腺功能亢进和骨软化症。治疗目标应侧重于避免25(OH)D血清水平<30nmol/L,目标是达到>50nmol/L水平,以避免维生素D缺乏带来的不利影响。为了减少老年人骨折,充足的维生素D和钙是必要的。
参考文献
1.Giustina A, Adler RA, Binkley N, etal. Controversies in Vitamin D: summary statement from an international conference.J. Clin. Endocrinol. Metab.2019;104:234–240.
2.Sempos CT, Heijboer AC, Bikle DD,etal. Vitamin D assays and the definition of hypovitaminosis D: Results from the first international conference on controversies in vitamin D.Br. J. Clin. Pharmacol.2018;84:2194–2207.
3.Ebeling PR, Adler RA, Jones G, etal. Management of endocrine disease: Therapeutics of vitamin D.Eur. J. Endocrinol.2018;179:R239–R259.
4.Bouillon R, Marcocci C, Carmeliet G,etal. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions.Endocr. Rev.2019;40:1109–1151.
5.Giustina A, Adler RA, Binkley N,etal. Consensus statement from 2nd International Conference on Controversies in Vitamin D.Rev. Endocr. Metab. Disord.2020;21:89–116.
6.Giustina A, Bouillon R, Binkley N, etal. Controversies in Vitamin D: a statement from the third international conference.JBMR.2020;4:e10417.
7.Bilezikian JP, Formenti AM, Adler RA, etal. Vitamin D: dosing, levels, form, and route of administration: does one approach fit all.Rev. Endocr. Metab. Disord.2021;22:1201–1218.
8.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3.J. Clin. Invest.1985;76:1536–1538.
9.Holick MF, Chen TC, Lu Z, etal. Vitamin D and skin physiology: a D-lightful story.J. Bone Miner. Res.2007;22:V28–V33.
10.Chalcraft JR, Cardinal LM, Wechsler PJ, etal. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women.Nutrients.2020;12:2237.
11.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications.Endocr. Rev.2001;22:477–501.
12.de Jongh RT, van Schoor NM, Lips P. Changes in vitamin D endocrinology during aging in adults.Mol. Cell. Endocrinol.2017;453:144–150.
13.Chel VG, Ooms ME, Popp-Snijders C, etal, Meulemans CC, Lips P. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly.J. Bone Miner. Res.1998;13:1238–1242.
14.Chel VG, Ooms ME, Pavel S, etal. Prevention and treatment of vitamin D deficiency in Dutch psychogeriatric nursing home residents by weekly half-body UVB exposure after showering: a pilot study.Age Ageing.2011;40:211–124.
15.Mousavi SE, Amini H, Heydarpour P, etal. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms.Environ. Int.2019;122:67–90.
16.Passeron T, Bouillon R, Callender V, etal. Sunscreen photoprotection and vitamin D status.Br. J. Dermatol.2019;181:916–931.
17.Cutillas-Marco E, Fuertes-Prosper A, Grant WB, etal. Vitamin D deficiency in South Europe: effect of smoking and aging.Photodermatol. Photoimmunol. Photomed.2012;28:159–161.
18.Snijder MB, van Dam RM, Visser M, etal.P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women.J. Clin. Endocrinol. Metab.2005;90:4119–4123.
19.NCD Risk Factor Collaboration (NCD-RisC) Rising rural body-mass index is the main driver of the global obesity epidemic in adults.Nature.2019;569:260–264.
20.Reinders I, van Schoor NM, Deeg DJH, etal. Trends in lifestyle among three cohorts of adults aged 55-64 years in 1992/1993, 2002/2003 and 2012/2013.Eur. J. Public Health.2018;28:564–570.
21.van Dam RM, Snijder MB, Dekker JM, etal. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study.Am. J. Clin. Nutr.2007;85:755–761.
22.van den Heuvel EG, van Schoor N, de Jongh RT, etal. Cross-sectional study on different characteristics of physical activity as determinants of vitamin D status; inadequate in half of the population.Eur. J. Clin. Nutr.2013;67:360–365.
23.Vallejo MS, Blümel JE, Arteaga E, etal. Gender differences in the prevalence of vitamin D deficiency in a southern Latin American country: a pilot study.Climacteric.2020;23:410–416.
24.Sanghera DK, Sapkota BR, Aston CE, etal. Vitamin D status, gender differences, and cardiometabolic health disparities.Ann. Nutr. Metab.2017;70:79–87.
25.Z. Wu, C.A. Camargo Jr., I.R. Reid, etal, What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations. ? J. Steroid. Biochem. Mol. Biol.201, 105687 (2020).
26.Chapuy MC, Arlot ME, Duboeuf F, etal. Vitamin D3 and calcium to prevent hip fractures in elderly women.N. Engl. J. Med.1992;327:1637–1642.
27.Lips P, Chapuy MC, Dawson-Hughes B, etal. An international comparison of serum 25-hydroxyvitamin D measurements.Osteoporos. Int.1999;9:394–397.
28.Lips P, Duong T, Oleksik A, etal. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial.J. Clin. Endocrinol. Metab.2001;86:1212–1221.
29.Sohl E, de Jongh RT, Heymans MW, etal. Thresholds for serum 25(OH)D concentrations with respect to different outcomes.J. Clin. Endocrinol. Metab.2015;100:2480–2488.
30.Kuchuk NO, van Schoor NM, Pluijm SM, etal. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective.J. Bone Miner. Res.2009;24:693–701.
31.Reid IR, Horne AM, Mihov B, etal. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial.J. Intern. Med.2017;282:452–460.
32.Khaw KT, Stewart AW, Waayer D,etal. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial.Lancet Diabetes Endocrinol.2017;5:438–447.
33.Macdonald HM, Reid IR, Gamble GD, etal. 25-Hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial.J. Bone Miner. Res.2018;33:1464–1469.
34.de França NA, Camargo MB, Lazaretti-Castro M, etal. Dietary patterns and bone mineral density in Brazilian postmenopausal women with osteoporosis: a cross-sectional study.Eur. J. Clin. Nutr.2016;70:85–90.
35.Lips P, van Ginkel FC, Jongen MJ, etal, van der Vijgh WJ, Netelenbos JC. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects.Am. J. Clin. Nutr.1987;46:1005–1010.
36.Buttriss JL, Lanham-New SA, Steenson S, etal, Delamare G, Hoyland AE, Larsen L, Street LN, Mathers JC, Prentice A. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: current controversies and future perspectives: proceedings of the 2nd Rank Prize Funds Forum on vitamin D.Br. J. Nutr.2022;127:1567–1587.
37.Balk EM, Adam GP, Langberg VN, etal. International Osteoporosis Foundation Calcium Steering Committee.: Global dietary calcium intake among adults: a systematic review.Osteoporos. Int.2017;28:3315–3324.
38.Weaver CM, Alexander DD, Boushey CJ, etal. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation.Osteoporos. Int.2016;27:367–376.
39.Avenell A, Mcak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men.Cochrane Database Syst. Rev.2014;4:CD000227.
40.Yao P, Bennett D, Mafham M, etal. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis.JAMA Netw. Open.2019;2:e1917789.
41.Waterhouse M, Sanguineti E, Baxter C, etal. Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo-controlled D-Health trial.J. Cachexia Sarcopenia Muscle.2021;12:1428–1439.
42.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, etal. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial.JAMA Intern. Med.2016;176:175–183.
43.Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials.J. Bone Miner. Res.2021;36:1651–1660.
44.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, etal. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials.BMJ.2009;339:b3692.
45.Sanders KM, Stuart AL, Williamson EJ, etal. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial.JAMA.2010;303:1815–1822.
46.Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial.J. Steroid Biochem. Mol. Biol.2017;173:317–322.
47.Endo I, Inoue D, Mitsui T, etal. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors.Endocrinology.2003;144:5138–5144.
48.Beaudart C, Buckinx F, Rabenda V, etal. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials.J. Clin. Endocrinol. Metab.2014;99:4336–4345.
49.Garber AJ. Effects of parathyroid hormone on skeletal muscle protein and amino acid metabolism in the rat.J. Clin. Invest.1983;71:1806–1821.
50.Patten BM, Bilezikian JP, Mallette LE, etal. Neuromuscular disease in primary hyperparathyroidism.Ann. Intern. Med.1974;80:182–193.
51.Joborn C, Joborn H, Rastad J, etal. Maximal isokinetic muscle strength in patients with primary hyperparathyroidism before and after parathyroid surgery.Br. J. Surg.1988;75:77–80.
52.Bislev LS, Langagergaard Rødbro L, Sikjær T, etal. Effects of elevated parathyroid hormone levels on muscle health, postural stability and quality of life in vitamin D-insufficient healthy women: a cross-sectional study.Calcif. Tissue Int.2019;105:642–650.
53.Stein MS, Wark JD, Scherer SC, etal. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel.JAGS.1999;47:1195–1201.
54.Sambrook PN, Chen JS, March LM, etal. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study.J. Clin. Endocrinol. Metab.2004;89:5477–5481.
55.Pilz S, Kienreich K, Tomaschitz A, etal. Vitamin D and cardiovascular disease: update and outlook.Scand. J. Clin. Lab. Invest.2012;Suppl.:83–91.
56.Scragg R. The Vitamin D assessment (ViDA) study – design and main findings.J. Steroid Biochem. Mol. Biol.2020;198:105562.
57.Manson JE, Bassuk SS, Cook NR, etal. VITAL Research Group Vitamin D, Marine n-3 fatty acids, and primary prevention of cardiovascular disease current evidence.Circ. Res.2020;126:112–128. 58.Sluyter JD, Camargo CA, Jr., Stewart AW, etal. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: A randomized controlled trial substudy.J. Am. Heart Assoc.2017;6:e006802.
59.Manousaki D, Mokry LE, Ross S, etal. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease.Circ. Cardiovasc. Genet.2016;9:349–356.
60.Brøndum-Jacobsen P, Benn M, Afzal S, etal. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study.Int. J. Epidemiol.2015;44:651–661.
61.Vimaleswaran KS, Cavadino A, Berry DJ, etal. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study.Lancet Diabetes Endocrinol.2014;2:719–729.
62.Skaaby T, Husemoen LL, Martinussen T,etal. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach.PLoS One.2013;8:e57647.
63.Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses.Lancet Diabetes Endocrinol.2021;9:837–846.
64.Neale RE, Baxter C, Romero BD, etal. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality.Lancet Diabetes Endocrinol.2022;10:120–128.
65.Pittas AG, Dawson-Hughes B, Sheehan P, etal. D2d Research Group.: Vitamin D supplementation and prevention of type 2 diabetes.N. Engl. J. Med.2019;381:520–530.
66.Dawson-Hughes B, Staten MA, Knowler WC, etal, D2d Research Group. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the vitamin D and type 2 diabetes (D2d) study.Diabetes Care.2020;43:2916–2922.
67.Jorde R, Sollid ST, Svartberg J, etal. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes.J. Clin. Endocrinol. Metab.2016;101:1647–1655.
68.Kawahara T, Suzuki G, Inazu T, etal. Rationale and design of Diabetes prevention with active Vitamin D (DPVD): a randomised, double-blind, placebo-controlled study.BMJ Open.2016;6:e011183.
69.Kawahara. T.: Eldecalcitol, a vitamin D analogue, for diabetes prevention in impaired glucose tolerance (DPVD study). Diabetes Care.67(2018).
70.A.G. Pittas, R. Jorde, T. Kawahara, B. Dawson-Hughes. Vitamin D supplementation for prevention of type 2 diabetes mellitus: To d or not to D. ? J. Clin. Endocrinol. Metab.105, 3721–3733 (2020).
71.Barbarawi M, Zayed Y, Barbarawi O, etal. Effect of vitamin D supplementation on the incidence of diabetes mellitus.J. Clin. Endocrinol. Metab.2020;105:dgaa335.
72.Zhang Y, Tan H, Tang J, etal. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: A systematic review and meta-analysis.Diabetes Care.2020;43:1650–1658.
73.Matsuo LH, Confortin SC, Ceolin G, etal. Association between lower serum vitamin D (25-hydroxy-cholecalciferol) concentrations and cognitive impairment in older adults: data from a populational-based cohort study in a middle-income country.Public Health Nutr.2022;25:2507–2516.
74.Arosio B, Rossi PD, Ferri E, etal. Characterization of Vitamin D status in older persons with cognitive impairment.Nutrients.2022;14:1142.
75.Bivona G, Lo Sasso B, Gambino CM, etal. The role of vitamin D as a biomarker in alzheimer’s disease.Brain Sci.2021;11:334.
76.Perez L, Heim L, Sherzai A, Jaceldo-Siegl K. Nutrition and vascular dementia.J. Nutr. Health Aging.2012;16:319–324.
77.Hu J, Jia J, Zhang Y, etal. Effects of vitamin D3 supplementation on cognition and blood lipids: a 12-month randomised, double-blind, placebo-controlled trial.J. Neurol. Neurosurg. Psychiatry.2018;89:1341–1347.
78.Jia J, Hu J, Huo X, etal. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial.J. Neurol. Neurosurg. Psychiatry.2019;90:1347–1352.
79.Castle M, Fiedler N, Pop LC, etal. Three doses of vitamin d and cognitive outcomes in older women: a double-blind randomized controlled trial.J. Gerontol. A. Biol. Sci. Med. Sci.2020;75:835–842.
80.Yang T, Wang H, Xiong Y, etal. Vitamin D supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: a 12-month randomized controlled trial.J. Alzheimers Dis.2020;78:1509–1518.
81.Bouillon R, Manousaki D, Rosen C, etal. The health effects of vitamin D supplementation: evidence from human studies.Nat. Rev. Endocrinol.2022;18:96–110.
82.Barysch MJ, Hofbauer GF, Dummer R. Vitamin D, ultraviolet exposure, and skin cancer in the elderly.Gerontology.2010;56:410–413.
83.Maeda SS, Kunii IS, Hayashi LF, etal. Increases in summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in São Paulo, Brazil vary with age, gender and ethnicity.BMC Endocr. Disord.2010;10:12.
84.Samefors M, Tengblad A, Östgren CJ. Sunlight exposure and vitamin D levels in older people- an intervention study in Swedish nursing homes.J. Nutr. Health Aging.2020;24:1047–1052.
85.Sambrook PN, Cameron ID, Chen JS, etal. Does increased sunlight exposure work as a strategy to improve vitamin D status in the elderly: a cluster randomised controlled trial.Osteoporos. Int.2012;23:615–624.
86.Pinheiro MM, Schuch NJ, Genaro PS, etal. Nutrient intakes related to osteoporotic fractures in men and women–the Brazilian Osteoporosis Study (BRAZOS)Nutr. J.2009;8:6.
87.Buttriss JL, Lanham-New SA. Is a vitamin D fortification strategy needed?Nutr. Bull.2020;45:115–122.
88.Lips P, Cashman KD, Lamberg-Allardt C, etal. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society.Eur. J. Endocrinol.2019;180:P23–P54.
89.Cashman KD. Vitamin D Deficiency: defining, prevalence, causes, and strategies of addressing.Calcif. Tissue Int.2020;106:14–29.
90.Jääskeläinen T, Itkonen ST, Lundqvist A, etal. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data.Am. J. Clin. Nutr.2017;105:1512–1520.
91.Pilz S, Zittermann A, Trummer C, etal. Vitamin D testing and treatment: a narrative review of current evidence.Endocr. Connect.2019;8:R27–R43.
92.Cashman KD, Ritz C, Kiely M, Odin Collaborators. Improved dietary guidelines for vitamin D: Application of individual participant data (IPD)-level meta-regression analyses.Nutrients.2017;9:469.
93.https://www.fda.gov/media/151707/downloadAccessed 16 Sept 2021
94.Bilezikian JP, Bikle D, Hewison M,etal. Mechanisms in endocrinology: Vitamin D and COVID-19.Eur. J. Endocrinol.2020;183:R133–R147.
95.di Filippo L, Allora A, Doga M, etal. Vitamin D levels are associated with blood glucose and BMI in COVID-19 patients, predicting disease severity.J. Clin. Endocrinol. Metab.2022;107:e348–e360.
96.Chambers ES, Vukmanovic-Stejic M, Turner CT, etal. Vitamin D3 replacement enhances antigen-specific immunity in older adults.Immunother. Adv.2021;1:ltaa008.
97.Bouillon R, Quesada-Gomez JM. Vitamin D endocrine system and COVID-19.JBMR.2021;5:e10576.
98.Ulivieri FM, Banfi G, Camozzi V, etal. Vitamin D in the Covid-19 era: a review with recommendations from a G.I.O.S.E.G. expert panel.Endocrine.2021;72:597–603.

相关产品

相关阅读








 苏公网安备32011202001302
苏公网安备32011202001302